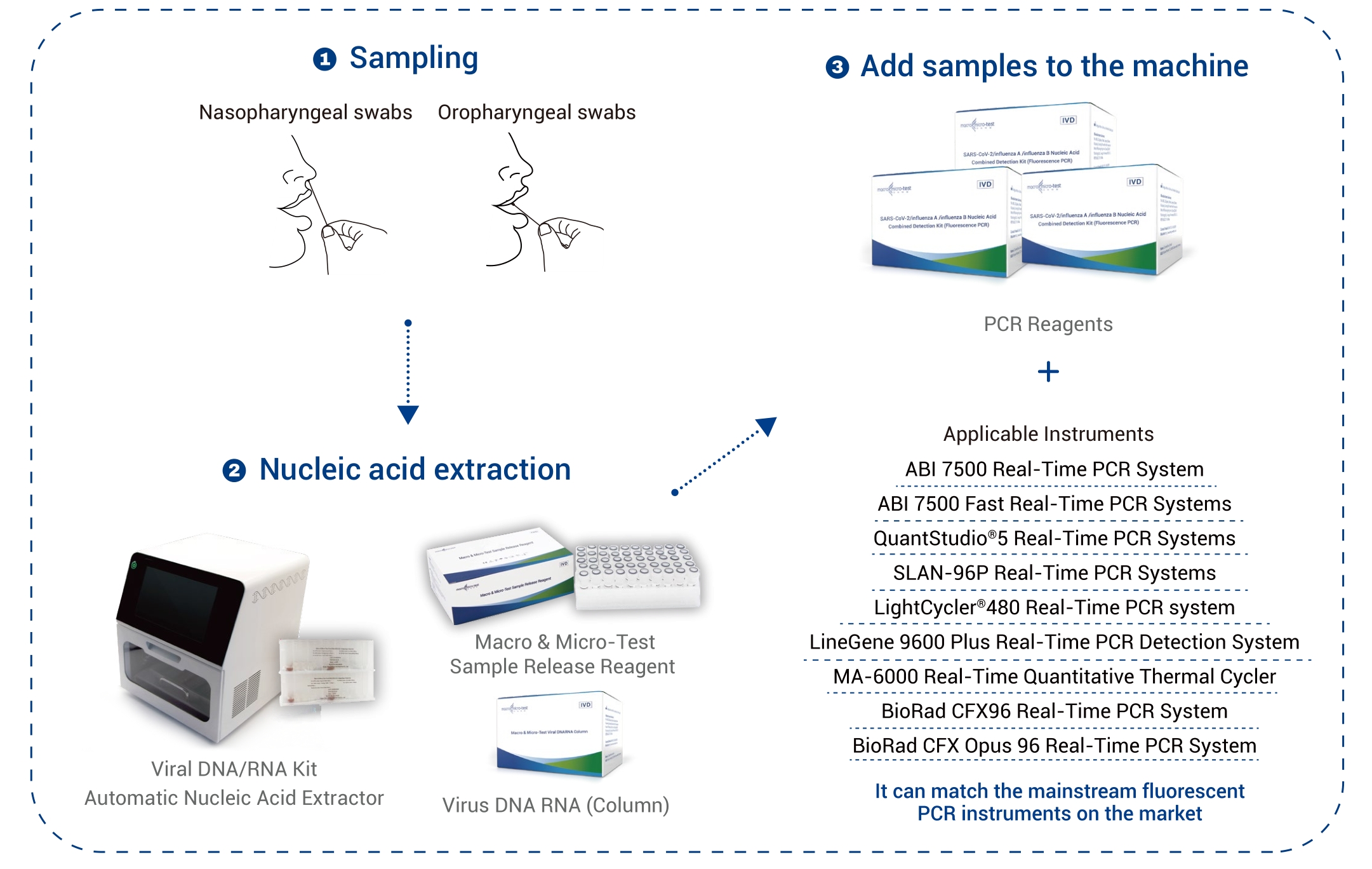
SARS-CoV-2/Gripp A/Gripp B
Produktnumm
HWTS-RT148-SARS-CoV-2/Gripp A/Gripp B Kombinéierten Detektiounskit fir Nukleinsaier (Fluoreszenz-PCR)
Kanal
| Kanalnumm | PCR-Mix 1 | PCR-Mix 2 |
| FAM Kanal | ORF1ab-Gen | IVA |
| VIC/HEX Kanal | Intern Kontroll | Intern Kontroll |
| CY5 Kanal | N-Gen | / |
| ROX Kanal | E-Gen | IVB |
Technesch Parameteren
| Späicherung | -18℃ |
| Haltbarkeet | 12 Méint |
| Probetyp | Nasofaryngeal- an Oropharyngeal-Teststäbchen |
| Zil | SARS-CoV-2 dräi Ziler (Orf1ab-, N- an E-Genen) / Gripp A / Gripp B |
| Ct | ≤38 |
| CV | ≤10,0% |
| LoD | SARS-CoV-2: 300 Kopien/ml Grippevirus A: 500 Kopien/ml Grippevirus B: 500 Kopien/ml |
| Spezifizitéit | a) D'Resultater vum Kräiztest hunn gewisen, datt de Kit kompatibel war mam mënschleche Coronavirus SARSr-CoV, MERSr-CoV, HcoV-OC43, HcoV-229E, HcoV-HKU1, HCoV-NL63, respiratoresche Synzytialvirus A a B, Parainfluenzavirus 1, 2 an 3, Rhinovirus A, B an C, Adenovirus 1, 2, 3, 4, 5, 7 an 55, mënschleche Metapneumovirus, Enterovirus A, B, C an D, mënschleche zytoplasmatesche Lungenvirus, EB-Virus, Masernvirus, mënschleche Cytomegalovirus, Rotavirus, Norovirus, Mumpsvirus, Varicella-Zostervirus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella, Pertussis, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Klebsiella pneumoniae, Mycobacterium tuberculosis, Aspergillus fumigatus, Candida albicans, Candida glabrata Et gouf keng Kräizreaktioun tëscht Pneumocystis yersini a Cryptococcus neoformans festgestallt. b) Anti-Interferenzfäegkeet: ausgewählte Mucin (60 mg/mL), 10% (V/V) mënschlecht Blutt, Diphenylephrin (2 mg/mL), Hydroxymethylzolin (2 mg/mL), Natriumchlorid (mat Konservéierungsmëttel) (20 mg/mL), Beclomethason (20 mg/mL), Dexamethason (20 mg/mL), Flunison (20 μg/mL), Triamcinolonacetonid (2 mg/mL), Budesonid (2 mg/mL), Mometason (2 mg/mL), Fluticason (2 mg/mL), Histaminhydrochlorid (5 mg/mL), α-Interferon (800 IU/mL), Zanamivir (20 mg/mL), Ribavirin (10 mg/mL), Oseltamivir (60 ng/mL), Pramivir (1 mg/mL), Lopinavir (500 mg/mL), Ritonavir (60 mg/mL), Mupirocin (20 mg/mL), Azithromycin (1 mg/mL), Ceproten (40 μg/mL), Meropenem (200 mg/mL), Levofloxacin (10 μg/mL) an Tobramycin (0,6 mg/mL). D'Resultater hunn gewisen, datt déi stéierend Substanzen an den uewe genannten Konzentratioune keng Stéierungsreaktioun op d'Detektiounsresultater vu Pathogenen haten. |
| Uwendbar Instrumenter | Applied Biosystems 7500 Echtzäit-PCR-Systemer Applied Biosystems 7500 Schnell Echtzäit-PCR-Systemer SLAN ®-96P Echtzäit-PCR-Systemer QuantStudio™ 5 Echtzäit-PCR-Systemer Liichtzyklus®480 Echtzäit-PCR-System LineGene 9600 Plus Echtzäit-PCR-Detektiounssystem MA-6000 Echtzäit Quantitativen Thermozykléierer BioRad CFX96 Echtzäit-PCR-System BioRad CFX Opus 96 Echtzäit-PCR-System |
Total PCR-Léisung